NANOCOMPOSITE AND MECHANICALLY ALLOYED REACTIVE MATERIALS AS ENERGETIC ADDITIVES IN CHEMICAL OXYGEN GENERATORS
Marco A. Machado, Daniel A. Rodriguez, Yasmine Aly, Mirko Schoenitz, Edward L. Dreizin, and Evgeny Shafirovich
Chemical oxygen generators are widely used for aircraft, spacecraft, submarines, and mine rescue. Oxygen-generating compositions typically include alkali metal chlorate or perchlorate that decomposes at increased temperatures, a transition-metal oxide as a decomposition catalyst, and a metal fuel that reacts with part of the produced oxygen to provide heat for a self-sustained propagation of the decomposition/combustion wave. To increase the oxygen yield per unit mass, it is of interest to minimize the amount of metal fuel, but decreasing its content leads to pulsating combustion and undesired fluctuations of the oxygen flow rate. The present paper explores the feasibility of replacing iron and tin, currently used in oxygen generators, with reactive materials, produced by arrested reactive milling and by mechanical alloying. Because of their high energy density, easy ignition, and good storability, these materials have the potential to improve the performance characteristics of oxygen generators. Thermodynamic calculations for combustion of sodium chlorate mixed with various reactive materials identified the most attractive additives providing high temperatures and high oxygen yield. Experiments on combustion of sodium chlorate-based mixtures with nanoscale cobalt oxide catalyst and the most promising energetic additives were conducted in an argon environment, using laser ignition. Infrared video recording was used to investigate the thermal wave propagation over the mixture pellet. The experiments have shown that mechanically alloyed Al/Mg (1:1 mass ratio) material is a promising alternative to iron and tin, because significantly smaller amounts of this additive are needed for a steady propagation of the combustion wave.
1. Introduction
Chemical oxygen generators, also called self-contained oxygen generators (SCOG) and oxygen candles, are widely used for aircraft, spacecraft, submarines, and mine rescue. Oxygen-generating compositions typically include alkali metal chlorate or perchlorate (e.g., sodium chlorate, NaClO3) that decomposes at increased temperatures with release of oxygen. Increased temperatures are achieved by an exothermic reaction between an added metal fuel (e.g., iron or tin) and part of the product oxygen. The compositions also include a transition-metal oxide catalyst (e.g., cobalt oxide, Co3O4) that significantly decreases the decomposition temperature of the oxygen source. As a result, oxygen is generated through a self-sustained propagation of the decomposition/combustion wave over the generator’s chemical core.
In oxygen generators based on sodium chlorate, the metal fuel provides combustion temperatures of 400‒800 °C (decomposition of NaClO3 is exothermic and contributes to the total heat release). The minimum limit is determined by the necessity to fully decompose sodium chlorate and ensure a self-sustained combustion, while the maximum temperature is limited by the melting point of sodium chloride (NaCl) byproduct (800.7 °C), melting of which hinders a uniform transport of the released oxygen outward and makes the process highly unsteady. Combustion of the metal fuel, however, consumes part of the product oxygen, thus lowering the oxygen yield per unit mass. It is of interest to minimize the amount of metal fuel, but decreasing its content leads to undesired pulsations of the combustion wave and oxygen flow rate.
The present paper investigates an approach to the design of efficient and safe chemical gas generators that exploits recently developed reactive materials. Specifically, the paper explores the feasibility of replacing iron and tin, currently used in oxygen generators based on sodium chlorate, with reactive materials produced by arrested reactive milling (ARM) and by mechanical alloying.
Nanocomposite reactive materials obtained by ARM contain fully-dense, micron scale particles in which components are mixed on the scale of about 100 nm, but are not chemically bound. These materials include compositions combining metals and oxidizers as well as compositions that take advantage of uniform nano-scale mixing between components reacting exothermically and not requiring oxygen (e.g., intermetallic or metal-metalloid composites).
Mechanically alloyed powders were shown to be more reactive fuels compared to regular alloys or pure metals. Their developed surface area accelerates ignition, which is also assisted by weakly exothermic, subsolidus intermetallic reactions. In addition, combustion may be optimized by finely tuned elemental compositions.
The nanocomposite and mechanically alloyed reactive materials have the potential to improve the performance characteristics of chemical oxygen generators. Because of a higher gas yield, more controlled heat release, and a smaller amount of energetic additives, both efficiency and process stability of chemical oxygen generators could be improved.
The objective of the present paper is to identify the reactive materials that are the most efficient additives to sodium chlorate in providing stable combustion and high oxygen yield. First, a broad range of reactive materials, including various thermites, intermetallics, and metal/nonmetal mixtures that had been synthesized by ARM, were screened through thermodynamic calculations for combustion of oxygen-generating compositions based on sodium chlorate. Then, several thermodynamically promising materials were tested experimentally as components of combustible oxygen-generating mixtures.
In oxygen generators based on sodium chlorate, the metal fuel provides combustion temperatures of 400‒800 °C (decomposition of NaClO3 is exothermic and contributes to the total heat release). The minimum limit is determined by the necessity to fully decompose sodium chlorate and ensure a self-sustained combustion, while the maximum temperature is limited by the melting point of sodium chloride (NaCl) byproduct (800.7 °C), melting of which hinders a uniform transport of the released oxygen outward and makes the process highly unsteady. Combustion of the metal fuel, however, consumes part of the product oxygen, thus lowering the oxygen yield per unit mass. It is of interest to minimize the amount of metal fuel, but decreasing its content leads to undesired pulsations of the combustion wave and oxygen flow rate.
The present paper investigates an approach to the design of efficient and safe chemical gas generators that exploits recently developed reactive materials. Specifically, the paper explores the feasibility of replacing iron and tin, currently used in oxygen generators based on sodium chlorate, with reactive materials produced by arrested reactive milling (ARM) and by mechanical alloying.
Nanocomposite reactive materials obtained by ARM contain fully-dense, micron scale particles in which components are mixed on the scale of about 100 nm, but are not chemically bound. These materials include compositions combining metals and oxidizers as well as compositions that take advantage of uniform nano-scale mixing between components reacting exothermically and not requiring oxygen (e.g., intermetallic or metal-metalloid composites).
Mechanically alloyed powders were shown to be more reactive fuels compared to regular alloys or pure metals. Their developed surface area accelerates ignition, which is also assisted by weakly exothermic, subsolidus intermetallic reactions. In addition, combustion may be optimized by finely tuned elemental compositions.
The nanocomposite and mechanically alloyed reactive materials have the potential to improve the performance characteristics of chemical oxygen generators. Because of a higher gas yield, more controlled heat release, and a smaller amount of energetic additives, both efficiency and process stability of chemical oxygen generators could be improved.
The objective of the present paper is to identify the reactive materials that are the most efficient additives to sodium chlorate in providing stable combustion and high oxygen yield. First, a broad range of reactive materials, including various thermites, intermetallics, and metal/nonmetal mixtures that had been synthesized by ARM, were screened through thermodynamic calculations for combustion of oxygen-generating compositions based on sodium chlorate. Then, several thermodynamically promising materials were tested experimentally as components of combustible oxygen-generating mixtures.
2. Thermodynamic Calculations
Thermodynamic calculations of the adiabatic flame temperatures and combustion product compositions were conducted. Table 1 shows the amounts of 25 binary mixtures that need to be added to sodium chlorate to provide the adiabatic flame temperatures of 600, 700, and 800 °C. Table 1 also shows the mass fraction of molecular oxygen (O2) in the combustion products.
For comparison, Table 1 presents the values obtained for iron and tin, which are currently used in chemical oxygen generators. This allows one to readily downselect the mixtures that provide higher theoretical temperatures and O2 yield compared to the current formulations.
Further, the values obtained for pure aluminum and magnesium are presented. Aluminum is not used in chemical oxygen generators because it does not ignite at relatively low temperatures occurring in these generators and, in addition, does not show any catalytic effect on the decomposition of sodium chlorate. Because of handling and stability problems (quick aging in oxidizing and moist environments and high fire hazard), magnesium is not used either. However, Al/Mg materials obtained by mechanical alloying are promising additives because they ignite easier than Al and, at the same time, are expected to be substantially more stable and less hazardous than pure Mg.
In Table 1, the mixtures containing smaller overall weight % of additive compared to the formulation with Fe are most promising. Among the metal-metal and metal-metalloid reactive materials, B/Ti and Al/Ti mixtures provide the best results, while Al/NaNO3 is the best among nanocomposite thermites. Although calculations were not performed for various Al/Mg alloys, considering the results obtained for pure Al and Mg, such alloys are expected to perform similarly to B/Ti and Al/Ti composites. Thermodynamically, all these additives are more efficient than iron or tin. More specifically, a lower concentration of the additive provides the same adiabatic combustion temperature and, simultaneously, a higher oxygen yield.
Note that the combustion products predicted to form by the thermodynamic calculations are fully oxidized. There are no metals or unoxidized compounds such as TiB2 and AlTi in the predicted products. At the same time, the idea of using reactive materials is based on the initial exothermic reactions between the additive’s components that kick-start combustion. The generated intermetallic or other phases are expected to oxidize in the subsequent reaction with the produced oxygen.
Thermodynamic calculations were also conducted for several mixtures that included an additional 3 wt % of Co3O4, which was used as a catalyst in the present experiments. Those calculations did not lead to a noticeable deviation from the data presented in Table 1.
For comparison, Table 1 presents the values obtained for iron and tin, which are currently used in chemical oxygen generators. This allows one to readily downselect the mixtures that provide higher theoretical temperatures and O2 yield compared to the current formulations.
Further, the values obtained for pure aluminum and magnesium are presented. Aluminum is not used in chemical oxygen generators because it does not ignite at relatively low temperatures occurring in these generators and, in addition, does not show any catalytic effect on the decomposition of sodium chlorate. Because of handling and stability problems (quick aging in oxidizing and moist environments and high fire hazard), magnesium is not used either. However, Al/Mg materials obtained by mechanical alloying are promising additives because they ignite easier than Al and, at the same time, are expected to be substantially more stable and less hazardous than pure Mg.
In Table 1, the mixtures containing smaller overall weight % of additive compared to the formulation with Fe are most promising. Among the metal-metal and metal-metalloid reactive materials, B/Ti and Al/Ti mixtures provide the best results, while Al/NaNO3 is the best among nanocomposite thermites. Although calculations were not performed for various Al/Mg alloys, considering the results obtained for pure Al and Mg, such alloys are expected to perform similarly to B/Ti and Al/Ti composites. Thermodynamically, all these additives are more efficient than iron or tin. More specifically, a lower concentration of the additive provides the same adiabatic combustion temperature and, simultaneously, a higher oxygen yield.
Note that the combustion products predicted to form by the thermodynamic calculations are fully oxidized. There are no metals or unoxidized compounds such as TiB2 and AlTi in the predicted products. At the same time, the idea of using reactive materials is based on the initial exothermic reactions between the additive’s components that kick-start combustion. The generated intermetallic or other phases are expected to oxidize in the subsequent reaction with the produced oxygen.
Thermodynamic calculations were also conducted for several mixtures that included an additional 3 wt % of Co3O4, which was used as a catalyst in the present experiments. Those calculations did not lead to a noticeable deviation from the data presented in Table 1.
Table 1. Calculated amounts of additives to sodium chlorate that provide adiabatic flame temperatures of 600, 700, and 800 °C and respective mass fractions of molecular oxygen in the products. Materials are shown in the order of increasing fraction of the additive at 800 °C.
3. Experimental
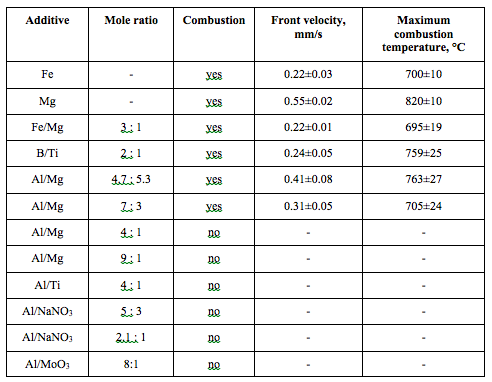
Combustion experiments were conducted with the following reactive material additives:
· Pure metals: Fe and Mg
· Mechanically alloyed powders: Fe/Mg (3:1), Al/Mg (4.7:5.3), Al/Mg (7:3), Al/Mg (4:1), and Al/Mg (9:1)
· Reactive metal-metal composites: Al/Ti (4:1)
· Reactive metal-metalloid composites: B/Ti (2:1)
· Nanocomposite thermites: Al/NaNO3 (5:3), Al/NaNO3 (2.1:1), and Al/MoO3 (8:1)
Here, the mole ratios are indicated. Note that for Al/Mg, 4.7:5.3 mole ratio corresponds to 1:1 mass ratio.
· Pure metals: Fe and Mg
· Mechanically alloyed powders: Fe/Mg (3:1), Al/Mg (4.7:5.3), Al/Mg (7:3), Al/Mg (4:1), and Al/Mg (9:1)
· Reactive metal-metal composites: Al/Ti (4:1)
· Reactive metal-metalloid composites: B/Ti (2:1)
· Nanocomposite thermites: Al/NaNO3 (5:3), Al/NaNO3 (2.1:1), and Al/MoO3 (8:1)
Here, the mole ratios are indicated. Note that for Al/Mg, 4.7:5.3 mole ratio corresponds to 1:1 mass ratio.
3.1 Emissivity Measurements
For temperature measurements with the infrared camera, the pellet emissivity should be known. To determine the emissivities of the tested pellets, special experiments were conducted. Black-body models were fabricated for several tested materials. For each model, two identical pellets (diameter: 13 mm, length: 13 mm) were prepared and a hemispherical cavity (diameter: 9.5 mm) was made using a ball nose end mill (by hand) in each of them. Then, the two pellets were attached to each other applying an adhesive tape over the cylindrical surface, so that a spherical cavity was obtained inside the resulting composite body. A 1.2-mm diameter channel was drilled to connect one end of the composite pellet with the cavity (Fig. 1).
The black body model was placed on a hot plate (Thermo Scientific HP2305B) and heated. The temperature at the center of the channel (i.e., the temperature of material at the inner surface of the cavity) was measured using the infrared camera and software, with the assumption that the emissivity is equal to 1. At the selected power of the hot plate, the temperature in the cavity was stabilized at around 90 °C. After that, the temperatures of the cavity and of the external pellet surface at its flat end near the channel were recorded and compared to each other.
For an accurate determination of the emissivity from these experiments, the temperature difference between the cavity and the external pellet surface should be as small as possible. It is generally accepted to neglect the temperature distribution inside the heated body if the Biot number is less than 0.1. The Biot number was estimated using the formula: Bi = h · k-1 · Lc where h is the heat transfer coefficient, k is the thermal conductivity of the pellet, and Lc is the characteristic size. The specific heat of NaClO3 is 0.95 J·g-1·K-1 at 25 °C and 1.05 J·g-1·K-1 at 90 °C. The addition of 3 wt % Co3O4 and 5 wt % of an energetic material only slightly influences the specific heat of the pellet. Using the average specific heat of NaClO3, 1 J·g-1·K-1, the measured thermal diffusivity of NaClO3-based pellets, 0.3 mm2·s-1, and the pellet density, 2 g·cm-3, the thermal conductivity was estimated to be 0.6 J·m-1·K-1. The characteristic size, calculated as the volume-to surface ratio, where the cavity volume was subtracted from the pellet volume, was equal to 2.4 mm. The heat transfer coefficient, calculated using the formulas for natural convection around a horizontal cylinder, was equal to 13 W·m-1·K-1. At the aforementioned parameters, the Biot number is equal to 0.052.
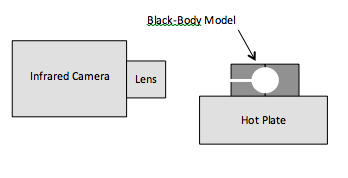
Since the temperature decreases with increasing the distance from the hot plate (Fig. 2), the measurements were conducted along an isothermal line that passed through the hole. Rectangular regions in the channel (box 4 in Fig. 2) and along the isothermal line (boxes 3 and 5 in Fig. 2) were selected on the images and the average temperature was determined for each region. The temperature of the flat surface along the isothermal line, measured with the assumption of the emissivity equal to 1, was lower than that of the cavity by 8–10 °C. In reality, due to the low Biot number of the tested pellets, the temperature difference between the internal surface of the cavity and the external surface of the pellet is negligible. Thus, the lower value of the measured temperature of the external surface is explained by the lower emissivity. Assuming that the emissivity of the “black-body” cavity is 1, the emissivity of the pellet surface was calculated.
The emissivities of five different pellet compositions (no additive, 3 wt% Al/Mg (7:3), 5 wt% Mg, 4 wt% Fe/Mg, and 5 wt% Fe/Mg) were determined based on 30 measurements for each, taken at different times after the temperature stabilization. The scatter of data for each composition was larger than the change due to changing the composition, which is understandable because the compositions were similar (with 92‒97 wt% NaClO3, 3 wt% Co3O4, and 0–5 wt% of the energetic additive). Averaging all the values produced an emissivity of 0.80 ± 0.05.
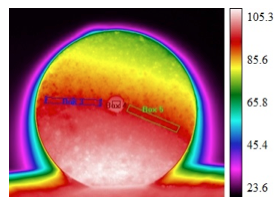
In addition, the emissivity measurements were conducted using the melting point of sodium chlorate (248 ºC [8]). A pellet with Al/Mg (7:3) additive was broken and several pieces were placed onto the hot plate and heated up to achieve melting of NaClO3. Figure 3 shows an infrared image of the heated pieces and the temperature profile along the vertical line in the image. The temperatures were measured in the range from 100 to 300 °C, with the assumption that the emissivity is equal to 0.8. The bottom of the image (where the temperature is lower than the maximum) corresponds to the hot plate and should be ignored. It is seen that the profile includes a distinct plateau at about 250 °C, which clearly indicates melting. This remarkable agreement with the melting point of NaClO3 confirms that the emissivity of this pellet was very close to 0.8.
Based on the emissivity measurements, in the analysis of combustion experiments, it was assumed that the emissivity of all the samples was 0.8, independently of the pellet composition. It was also assumed that the emissivity remains constant for the range of temperatures observed in the experiments (typically less than 800 °C).
The black body model was placed on a hot plate (Thermo Scientific HP2305B) and heated. The temperature at the center of the channel (i.e., the temperature of material at the inner surface of the cavity) was measured using the infrared camera and software, with the assumption that the emissivity is equal to 1. At the selected power of the hot plate, the temperature in the cavity was stabilized at around 90 °C. After that, the temperatures of the cavity and of the external pellet surface at its flat end near the channel were recorded and compared to each other.
For an accurate determination of the emissivity from these experiments, the temperature difference between the cavity and the external pellet surface should be as small as possible. It is generally accepted to neglect the temperature distribution inside the heated body if the Biot number is less than 0.1. The Biot number was estimated using the formula: Bi = h · k-1 · Lc where h is the heat transfer coefficient, k is the thermal conductivity of the pellet, and Lc is the characteristic size. The specific heat of NaClO3 is 0.95 J·g-1·K-1 at 25 °C and 1.05 J·g-1·K-1 at 90 °C. The addition of 3 wt % Co3O4 and 5 wt % of an energetic material only slightly influences the specific heat of the pellet. Using the average specific heat of NaClO3, 1 J·g-1·K-1, the measured thermal diffusivity of NaClO3-based pellets, 0.3 mm2·s-1, and the pellet density, 2 g·cm-3, the thermal conductivity was estimated to be 0.6 J·m-1·K-1. The characteristic size, calculated as the volume-to surface ratio, where the cavity volume was subtracted from the pellet volume, was equal to 2.4 mm. The heat transfer coefficient, calculated using the formulas for natural convection around a horizontal cylinder, was equal to 13 W·m-1·K-1. At the aforementioned parameters, the Biot number is equal to 0.052.
Since the temperature decreases with increasing the distance from the hot plate (Fig. 2), the measurements were conducted along an isothermal line that passed through the hole. Rectangular regions in the channel (box 4 in Fig. 2) and along the isothermal line (boxes 3 and 5 in Fig. 2) were selected on the images and the average temperature was determined for each region. The temperature of the flat surface along the isothermal line, measured with the assumption of the emissivity equal to 1, was lower than that of the cavity by 8–10 °C. In reality, due to the low Biot number of the tested pellets, the temperature difference between the internal surface of the cavity and the external surface of the pellet is negligible. Thus, the lower value of the measured temperature of the external surface is explained by the lower emissivity. Assuming that the emissivity of the “black-body” cavity is 1, the emissivity of the pellet surface was calculated.
The emissivities of five different pellet compositions (no additive, 3 wt% Al/Mg (7:3), 5 wt% Mg, 4 wt% Fe/Mg, and 5 wt% Fe/Mg) were determined based on 30 measurements for each, taken at different times after the temperature stabilization. The scatter of data for each composition was larger than the change due to changing the composition, which is understandable because the compositions were similar (with 92‒97 wt% NaClO3, 3 wt% Co3O4, and 0–5 wt% of the energetic additive). Averaging all the values produced an emissivity of 0.80 ± 0.05.
In addition, the emissivity measurements were conducted using the melting point of sodium chlorate (248 ºC [8]). A pellet with Al/Mg (7:3) additive was broken and several pieces were placed onto the hot plate and heated up to achieve melting of NaClO3. Figure 3 shows an infrared image of the heated pieces and the temperature profile along the vertical line in the image. The temperatures were measured in the range from 100 to 300 °C, with the assumption that the emissivity is equal to 0.8. The bottom of the image (where the temperature is lower than the maximum) corresponds to the hot plate and should be ignored. It is seen that the profile includes a distinct plateau at about 250 °C, which clearly indicates melting. This remarkable agreement with the melting point of NaClO3 confirms that the emissivity of this pellet was very close to 0.8.
Based on the emissivity measurements, in the analysis of combustion experiments, it was assumed that the emissivity of all the samples was 0.8, independently of the pellet composition. It was also assumed that the emissivity remains constant for the range of temperatures observed in the experiments (typically less than 800 °C).
|
Fig. 1. Schematic of the experiment for measuring the pellet emissivity.
Fig 2. Infrared image of the black-body model heated on a hot plate. The scale shows temperature in °C.
|
Fig. 3. An infrared image of pieces of the mixture with 5 wt% Al/Mg (7:3) additive heated on a hot plate (the scale shows temperature in °C) and the temperature profile along the vertical line (downward) in the image.
|
4. Results
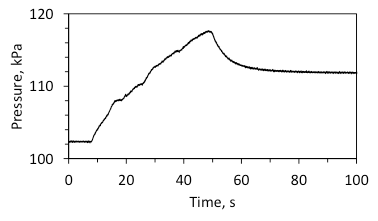
Figure 4 shows a typical pressure rise for an experiment where steady combustion occurred. The pressure gradually increases and then decreases to a stable value due to heat transfer to the chamber walls.
Based on the pressure increase, the mass of the evolved oxygen was calculated for each experiment, assuming the ideal gas behavior. Also, the oxygen mass was calculated based on the mass of decomposed sodium chlorate, assuming that each mole of NaClO3 decomposes to 1 mol of NaCl and 1.5 mol of O2. On average, the mass of oxygen determined from the pressure rise was less by 2.95% than that determined from the NaClO3 mass. Taking into consideration that small pieces of the initial material were typically found in the chamber after experiments, the obtained results are in good agreement.
Multiple experiments were performed for each of the 12 different compositions (145 experiments in total).
For iron, 5 wt% Fe was needed for a self-sustained combustion. The front propagated with an average velocity of 0.22 mm/s, with a maximum combustion temperature of 700 °C.
For direct comparisons with iron, all the mixtures were tested for ignition with 5 wt% of the additive. Table 2 summarizes the results of these experiments. The column “Combustion” indicates whether the mixture exhibits a self-sustained propagation of the combustion front. The fact that significantly larger amounts of additives are required for achieving thermodynamically predicted combustion temperatures is explained by heat losses. In addition to experiments presented in Table 2, for selected materials, experiments were performed with lower amounts of additives.
For Mg, both the maximum temperature and the front velocity were significantly higher than for Fe.
For Fe/Mg, the combustion characteristics were similar to those obtained with 5 wt% Fe. Also, 5 wt% is the minimum required concentration for combustion of the mixture with Fe/Mg additive. Thus, the addition of Mg to Fe (1:3 mole ratio) did not lead to any significant effect.
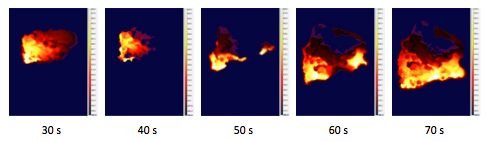
For B/Ti, the maximum temperature was significantly higher compared to Fe, but the front velocity was about the same. Numerous sparks were observed throughout the process (Fig. 5).
For Al/Mg (4.7:5.3), the maximum temperature was significantly higher than for Fe (though lower than for Mg) and the front velocity was twice the velocity for Fe.
For Al/Mg (7:3), the mixture burned slightly better than that with 5 wt% Fe. Also, 5 wt% was the minimum required concentration of the additive.
No ignition was observed for the mixtures with Al/Mg if the concentration of Mg was 20 mol% or less, for Al/Ti (4:1, both types of powder – prepared with stearic acid and with paraffin wax), Al/NaNO3 (both mole ratios), and Al/MoO3 (8:1).
Subsequent research was focused on Mg and Al/Mg (4.7:5.3) additives.
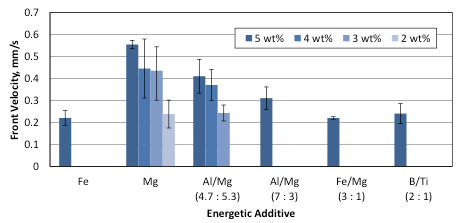
The mixture burned at only 2 wt% of Mg added, with the combustion characteristics of 0.24 mm/s and 720 °C, which are similar to those for the mixture with 5 wt% Fe. An increase in the additive’s concentration to 3 wt% resulted in the flame speed and temperature of 0.44 mm/s and 767 °C, respectively.
For Al/Mg (4.7:5.3), the mixture with 3 wt% additive burned with the front velocity being equal to 0.24 mm/s, i.e., as fast as the mixture with 5 wt% Fe. The measured temperature was equal to 685 °C. An increase in the concentration to 4 wt% enabled one to reach the flame speed and temperature of 0.37 mm/s and 767 °C, respectively.
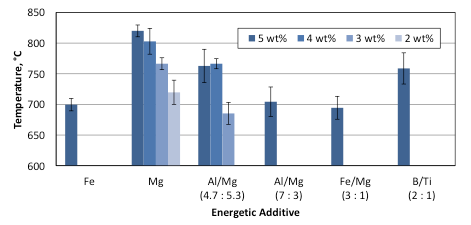
Figures 6 and 7 summarize the combustion front velocities and maximum combustion temperatures for the mixtures with Fe, Mg, Fe/Mg, B/Ti, and two Al/Mg additives. Analysis of the obtained experimental results shows that the replacement of Fe with the same or even smaller amount of Al/Mg (4.7:5.3) material increases both the combustion front velocity and the maximum temperature.
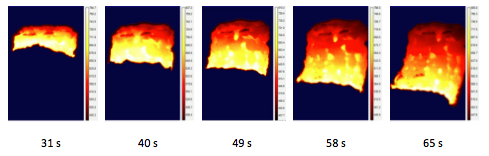
In addition, the replacement of Fe with Al/Mg (4.7:5.3) leads to a more uniform propagation of the combustion front. For illustration, Figures 8 and 9 show infrared images for the fragments of the combustion front propagation over the pellets with 5 wt % Fe and 5 wt % Al/Mg (4.7:5.3), respectively. It is seen that with the Fe additive, during the period from 46 s to 56 s, the front virtually stands at a fixed position and the temperature of the products is decreasing; then, the front is moving again and the temperature is increasing. Also, the front shape is irregular. In contrast, with Al/Mg additive, the front motion is rather uniform and the front is flatter.
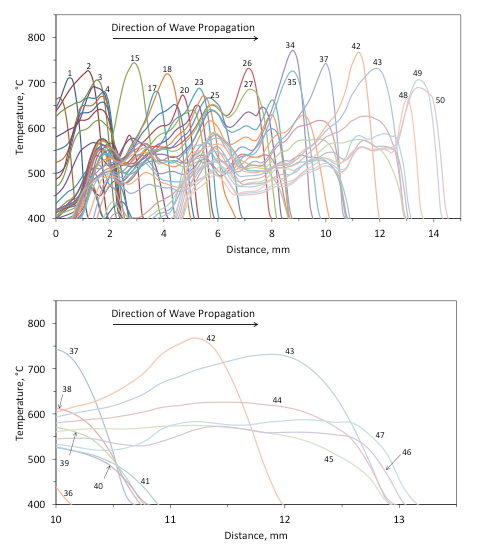
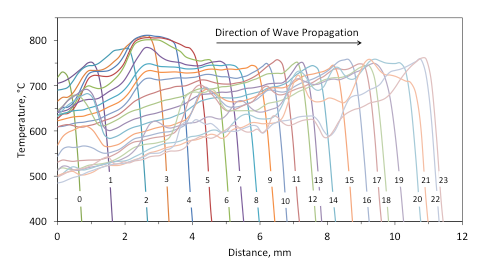
The pulsating and steady modes of the combustion front propagation can be seen clearly from the dynamics of temperature-distance profiles, obtained using infrared imaging. For example, Figure 10-a shows the thermal wave that propagates over a pellet that includes 5 wt % Fe (not the same sample as in Fig. 8). It is seen that significant pulsations in the front motion occur. For clarity, Figure 10-b presents a fragment of the plot shown in Fig. 10-a, with time labels for all curves. Only temperature profiles for instants of time from 36 s through 47 s are shown in this plot. It is seen that for 4 s (from 37 s to 41 s) the front almost does not move and the temperature decreases. Soon after that, the temperature increases and the front travels about 2 mm for 2 s (from 41 s to 43 s). Then, again, for 4 s (from 43 s to 47 s) the front virtually stands in a fixed location.
For comparison, Figure 11 shows the thermal wave that propagates over a pellet that includes 5 wt% of Al/Mg (4.7:5.3 mole ratio). It is seen that, in contrast with the Fe-containing mixture, the propagation is very uniform.
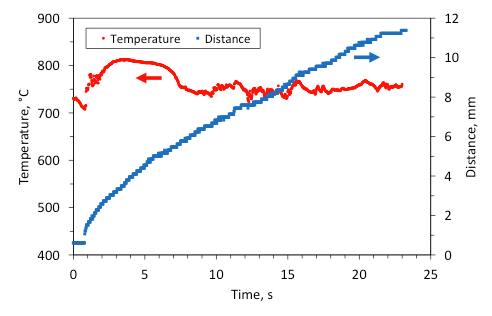
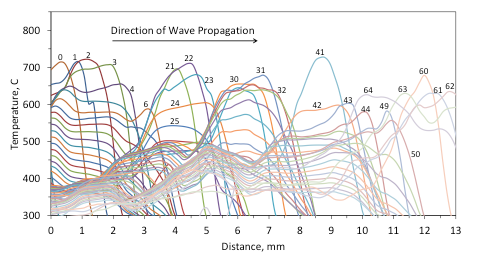
For clarity, for the same experiments that were reported in Figs. 10 and 11, Figures 12 and 13 show the time variation of the maximum temperature in the combustion wave and the distance traveled by the front as a function of time (a temperature of 400 °C in the front was taken to characterize its position). It is seen that for the mixture with 5 wt% Fe (Fig. 12), the maximum temperature fluctuates from 450 °C to 770 °C and the front motion includes complete stops and rapid jumps forward. The stops correlate with the periods of temperature fall, while the jumps occur when the temperature rises. On the contrary, for the mixture with 5 wt% Al/Mg (Fig. 13) the fluctuations are much smaller and the front propagates steadily.
As noted above, a decrease in Al/Mg concentration from 5 wt% to 3 wt% makes the combustion characteristics similar to those for the mixture with 5 wt% Fe. The combustion also becomes pulsating, as shown in Fig. 14.
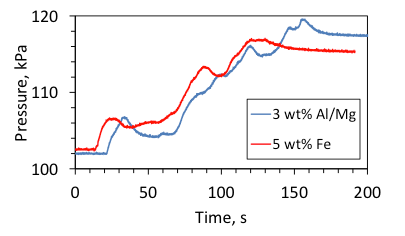
The front pulsations, such as those observed at 5 wt% Fe and 3 wt% Al/Mg, lead to the fluctuations of the oxygen flow rate, which are highly undesired in emergency oxygen generators. In the present experiments, the flow rate fluctuations result in the fluctuations of pressure in the reaction chamber. For example, Figure 15 shows the time variation of pressure during combustion of pellets with 5 wt% Fe and 3 wt% Al/Mg. The pressure pulsations did not occur during uniform combustion of the mixtures with 5 wt% Al/Mg.
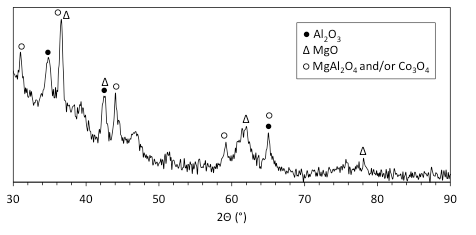
Figure 16 shows the XRD pattern of the combustion products of the mixture with 5 wt% Al/Mg (4.7:5.3 mole ratio) after removing NaCl. It is seen that the main peaks correspond to oxides of Al, Mg, and Co. Note that the main lines for Co3O4 are the same as for MgAl2O4 (2Θ: 31°, 37°, 45°, 59°, and 65°), so that it is impossible to distinguish these two phases in the diffraction pattern. The obtained results are in agreement with the experiments on combustion of mechanically alloyed Al/Mg particles in air, where XRD analysis showed MgO, Al2O3, and MgAl2O4 in the products. Also, the main lines for Al are at 39°, 45°, 65°, and 78°. The obtained diffraction pattern has a small peak at 39°, while three other lines overlap the peaks of other phases. Thus, it cannot be excluded that a small amount of free metallic Al is present in the products.
Based on the pressure increase, the mass of the evolved oxygen was calculated for each experiment, assuming the ideal gas behavior. Also, the oxygen mass was calculated based on the mass of decomposed sodium chlorate, assuming that each mole of NaClO3 decomposes to 1 mol of NaCl and 1.5 mol of O2. On average, the mass of oxygen determined from the pressure rise was less by 2.95% than that determined from the NaClO3 mass. Taking into consideration that small pieces of the initial material were typically found in the chamber after experiments, the obtained results are in good agreement.
Multiple experiments were performed for each of the 12 different compositions (145 experiments in total).
For iron, 5 wt% Fe was needed for a self-sustained combustion. The front propagated with an average velocity of 0.22 mm/s, with a maximum combustion temperature of 700 °C.
For direct comparisons with iron, all the mixtures were tested for ignition with 5 wt% of the additive. Table 2 summarizes the results of these experiments. The column “Combustion” indicates whether the mixture exhibits a self-sustained propagation of the combustion front. The fact that significantly larger amounts of additives are required for achieving thermodynamically predicted combustion temperatures is explained by heat losses. In addition to experiments presented in Table 2, for selected materials, experiments were performed with lower amounts of additives.
For Mg, both the maximum temperature and the front velocity were significantly higher than for Fe.
For Fe/Mg, the combustion characteristics were similar to those obtained with 5 wt% Fe. Also, 5 wt% is the minimum required concentration for combustion of the mixture with Fe/Mg additive. Thus, the addition of Mg to Fe (1:3 mole ratio) did not lead to any significant effect.
For B/Ti, the maximum temperature was significantly higher compared to Fe, but the front velocity was about the same. Numerous sparks were observed throughout the process (Fig. 5).
For Al/Mg (4.7:5.3), the maximum temperature was significantly higher than for Fe (though lower than for Mg) and the front velocity was twice the velocity for Fe.
For Al/Mg (7:3), the mixture burned slightly better than that with 5 wt% Fe. Also, 5 wt% was the minimum required concentration of the additive.
No ignition was observed for the mixtures with Al/Mg if the concentration of Mg was 20 mol% or less, for Al/Ti (4:1, both types of powder – prepared with stearic acid and with paraffin wax), Al/NaNO3 (both mole ratios), and Al/MoO3 (8:1).
Subsequent research was focused on Mg and Al/Mg (4.7:5.3) additives.
The mixture burned at only 2 wt% of Mg added, with the combustion characteristics of 0.24 mm/s and 720 °C, which are similar to those for the mixture with 5 wt% Fe. An increase in the additive’s concentration to 3 wt% resulted in the flame speed and temperature of 0.44 mm/s and 767 °C, respectively.
For Al/Mg (4.7:5.3), the mixture with 3 wt% additive burned with the front velocity being equal to 0.24 mm/s, i.e., as fast as the mixture with 5 wt% Fe. The measured temperature was equal to 685 °C. An increase in the concentration to 4 wt% enabled one to reach the flame speed and temperature of 0.37 mm/s and 767 °C, respectively.
Figures 6 and 7 summarize the combustion front velocities and maximum combustion temperatures for the mixtures with Fe, Mg, Fe/Mg, B/Ti, and two Al/Mg additives. Analysis of the obtained experimental results shows that the replacement of Fe with the same or even smaller amount of Al/Mg (4.7:5.3) material increases both the combustion front velocity and the maximum temperature.
In addition, the replacement of Fe with Al/Mg (4.7:5.3) leads to a more uniform propagation of the combustion front. For illustration, Figures 8 and 9 show infrared images for the fragments of the combustion front propagation over the pellets with 5 wt % Fe and 5 wt % Al/Mg (4.7:5.3), respectively. It is seen that with the Fe additive, during the period from 46 s to 56 s, the front virtually stands at a fixed position and the temperature of the products is decreasing; then, the front is moving again and the temperature is increasing. Also, the front shape is irregular. In contrast, with Al/Mg additive, the front motion is rather uniform and the front is flatter.
The pulsating and steady modes of the combustion front propagation can be seen clearly from the dynamics of temperature-distance profiles, obtained using infrared imaging. For example, Figure 10-a shows the thermal wave that propagates over a pellet that includes 5 wt % Fe (not the same sample as in Fig. 8). It is seen that significant pulsations in the front motion occur. For clarity, Figure 10-b presents a fragment of the plot shown in Fig. 10-a, with time labels for all curves. Only temperature profiles for instants of time from 36 s through 47 s are shown in this plot. It is seen that for 4 s (from 37 s to 41 s) the front almost does not move and the temperature decreases. Soon after that, the temperature increases and the front travels about 2 mm for 2 s (from 41 s to 43 s). Then, again, for 4 s (from 43 s to 47 s) the front virtually stands in a fixed location.
For comparison, Figure 11 shows the thermal wave that propagates over a pellet that includes 5 wt% of Al/Mg (4.7:5.3 mole ratio). It is seen that, in contrast with the Fe-containing mixture, the propagation is very uniform.
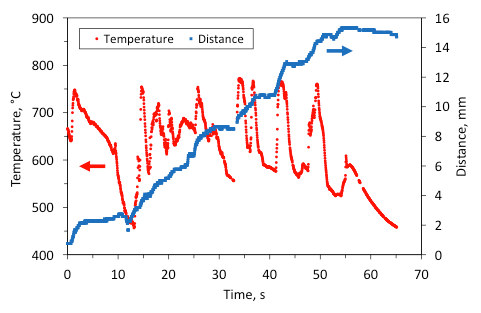
For clarity, for the same experiments that were reported in Figs. 10 and 11, Figures 12 and 13 show the time variation of the maximum temperature in the combustion wave and the distance traveled by the front as a function of time (a temperature of 400 °C in the front was taken to characterize its position). It is seen that for the mixture with 5 wt% Fe (Fig. 12), the maximum temperature fluctuates from 450 °C to 770 °C and the front motion includes complete stops and rapid jumps forward. The stops correlate with the periods of temperature fall, while the jumps occur when the temperature rises. On the contrary, for the mixture with 5 wt% Al/Mg (Fig. 13) the fluctuations are much smaller and the front propagates steadily.
As noted above, a decrease in Al/Mg concentration from 5 wt% to 3 wt% makes the combustion characteristics similar to those for the mixture with 5 wt% Fe. The combustion also becomes pulsating, as shown in Fig. 14.
The front pulsations, such as those observed at 5 wt% Fe and 3 wt% Al/Mg, lead to the fluctuations of the oxygen flow rate, which are highly undesired in emergency oxygen generators. In the present experiments, the flow rate fluctuations result in the fluctuations of pressure in the reaction chamber. For example, Figure 15 shows the time variation of pressure during combustion of pellets with 5 wt% Fe and 3 wt% Al/Mg. The pressure pulsations did not occur during uniform combustion of the mixtures with 5 wt% Al/Mg.
Figure 16 shows the XRD pattern of the combustion products of the mixture with 5 wt% Al/Mg (4.7:5.3 mole ratio) after removing NaCl. It is seen that the main peaks correspond to oxides of Al, Mg, and Co. Note that the main lines for Co3O4 are the same as for MgAl2O4 (2Θ: 31°, 37°, 45°, 59°, and 65°), so that it is impossible to distinguish these two phases in the diffraction pattern. The obtained results are in agreement with the experiments on combustion of mechanically alloyed Al/Mg particles in air, where XRD analysis showed MgO, Al2O3, and MgAl2O4 in the products. Also, the main lines for Al are at 39°, 45°, 65°, and 78°. The obtained diffraction pattern has a small peak at 39°, while three other lines overlap the peaks of other phases. Thus, it cannot be excluded that a small amount of free metallic Al is present in the products.
Table 2. Combustibility of mixtures with 5 wt% energetic additive.
|
Fig. 4. Typical time variation of pressure during steady combustion and cooling; the mixture with 5 wt% Al/Mg (4.7:5.3 mole ratio). Time zero was selected arbitrarily.
Fig. 6. Combustion front velocities of NaClO3-based mixtures with different energetic additives.
Fig. 8. Infrared images of combustion propagation over the pellet with 5 wt%Fe.
Fig. 10. Temperature-distance profiles at different instants of time 1 s apart for the pellet with 5 wt % Fe. The labels indicate time (s) from the start of propagation.
Fig. 13. Time variation of the maximum temperature in the combustion wave and the distance traveled by the front vs time for the mixture with 5 wt% Al/Mg.
Fig. 15. Time variation of pressure during combustion of the pellets with 5 wt% Fe and 3 wt% Al/Mg. Time zero was selected arbitrarily.
|
Fig. 5. Combustion of mixture with 5 wt % B/Ti additive.
Fig. 7. Maximum temperatures in the combustion front, measured at the pellet surface, for NaClO3-based mixtures with different energetic additives.
Fig. 9. Infrared images of combustion propagation over the pellet with 5 wt % Al/Mg (4.7:5.3 mole ratio).
Fig. 11. Temperature-distance profiles at different instants of time 1 s apart for the pellet with 5 wt% of Al/Mg (4.7:5.3 mole ratio). The labels indicate time (s) from the start of propagation.
Fig. 12. Time variation of the maximum temperature in the combustion wave and the distance traveled by the front vs time for the mixture with 5 wt% Fe.
Fig. 14. Temperature-distance profiles at different instants of time 1 s apart for the pellet with 3 wt% of Al/Mg (4.7:5.3 mole ratio). The labels indicate time (s) from the start of propagation.
Fig. 16. XRD pattern of the combustion products of the mixture with 5 wt% Al/Mg (4.7:5.3 mole ratio) after removing NaCl.
|
5. Discussion
The observed formation of sparks during combustion of the mixture with 5 wt% B/Ti is worthy of discussion. To our knowledge, this phenomenon has never been observed during combustion of sodium chlorate mixed with Fe, Sn, or other metals. During combustion of pyrotechnic compositions, the observed sparks are often tracks of single metal particles (or their agglomerates) burning in the atmosphere. In the present work, since the atmosphere was argon with only a small amount of oxygen generated by the combustion, the nature of the observed wide sparks was likely different. Apparently, fast combustion of B/Ti composite particles led to very high local temperatures and sharp decomposition of the surrounding sodium chlorate. The molten NaClO3 virtually boiled around B/Ti particles, leading to the ejection of large fragments of the mixture, which continued to burn and emit light during the flight.
Note that the maximum temperature during combustion of the mixture with 5 wt% B/Ti was significantly higher than for the mixture with Fe, but the front velocity was almost the same (see Table 2). Because of this and accounting for the undesired spark phenomenon, it is concluded that the replacement of Fe by B/Ti in chemical oxygen generators cannot not be recommended.
The failed attempts to ignite the mixtures with 5 wt% Al/NaNO3 and 5 wt% Al/MoO3 imply that the high exothermicity of the additive, if taken alone, does not guarantee that it will also work well in the mixture with sodium chlorate. The ignition of the additive should occur at a rate that is compatible with the ignition of sodium chlorate. It should be noted that to verify that the additive did not lose reactivity during storage, pellets that contained only Al/NaNO3 were prepared and tested. The observed vigorous combustion confirmed that the additive itself was not deteriorated.
Analysis of the obtained results (see Table 2) indicates that for successful operation in the sodium chlorate-based mixture, the energetic additive should include a metal that ignites in oxygen at relatively low temperatures (e.g., Mg or Ti). For example, the results for Al/Mg additives with different mixture ratios clearly show that a significant amount of Mg is required for the ignition of respective mixtures with NaClO3. Note, however, that in the mixtures with Mg/Al additives, Al is not an inert material. This is confirmed by XRD analysis, which indicated that both Al and Mg were oxidized during combustion of the mixture (see Fig. 16). Apparently, ignition of Mg creates local temperatures that are sufficient for the ignition of Al. This explanation is in agreement with prior results on the combustion of mechanically alloyed Al/Mg particles where it was shown that their ignition temperatures are close to those for Mg (much lower than for Al) and that Mg selectively burns first (stage I) followed by combustion of Al (stage II).
The results on combustion of NaClO3-based mixtures with Al/Mg (4.7:5.3 mole ratio) additive are remarkable. The mixture with 5 wt% Al/Mg provides a steady propagation of the combustion front, in contrast with pulsating combustion of the mixture with 5 wt% Fe. The mixture with 3 wt% Al/Mg behaves like the mixture with 5 wt% Fe. These results are quite understandable from the combustion theory standpoint. It is well known that a decrease in the exothermicity of the reaction leads to the transition from a steady propagation of the front to the pulsating combustion regime. The pulsating propagation of the combustion wave over a NaClO3-based oxygen generator has been demonstrated experimentally and numerically. Since Al/Mg additive is more energetic than Fe (see Table 1), steady combustion requires a smaller concentration of Al/Mg as compared with Fe. Note also that, in contrast with Mg, mechanically alloyed Al/Mg powder is expected to have much better storage characteristics. Thus, the use of this powder in chemical oxygen generators is worth of further consideration.
Note that the maximum temperature during combustion of the mixture with 5 wt% B/Ti was significantly higher than for the mixture with Fe, but the front velocity was almost the same (see Table 2). Because of this and accounting for the undesired spark phenomenon, it is concluded that the replacement of Fe by B/Ti in chemical oxygen generators cannot not be recommended.
The failed attempts to ignite the mixtures with 5 wt% Al/NaNO3 and 5 wt% Al/MoO3 imply that the high exothermicity of the additive, if taken alone, does not guarantee that it will also work well in the mixture with sodium chlorate. The ignition of the additive should occur at a rate that is compatible with the ignition of sodium chlorate. It should be noted that to verify that the additive did not lose reactivity during storage, pellets that contained only Al/NaNO3 were prepared and tested. The observed vigorous combustion confirmed that the additive itself was not deteriorated.
Analysis of the obtained results (see Table 2) indicates that for successful operation in the sodium chlorate-based mixture, the energetic additive should include a metal that ignites in oxygen at relatively low temperatures (e.g., Mg or Ti). For example, the results for Al/Mg additives with different mixture ratios clearly show that a significant amount of Mg is required for the ignition of respective mixtures with NaClO3. Note, however, that in the mixtures with Mg/Al additives, Al is not an inert material. This is confirmed by XRD analysis, which indicated that both Al and Mg were oxidized during combustion of the mixture (see Fig. 16). Apparently, ignition of Mg creates local temperatures that are sufficient for the ignition of Al. This explanation is in agreement with prior results on the combustion of mechanically alloyed Al/Mg particles where it was shown that their ignition temperatures are close to those for Mg (much lower than for Al) and that Mg selectively burns first (stage I) followed by combustion of Al (stage II).
The results on combustion of NaClO3-based mixtures with Al/Mg (4.7:5.3 mole ratio) additive are remarkable. The mixture with 5 wt% Al/Mg provides a steady propagation of the combustion front, in contrast with pulsating combustion of the mixture with 5 wt% Fe. The mixture with 3 wt% Al/Mg behaves like the mixture with 5 wt% Fe. These results are quite understandable from the combustion theory standpoint. It is well known that a decrease in the exothermicity of the reaction leads to the transition from a steady propagation of the front to the pulsating combustion regime. The pulsating propagation of the combustion wave over a NaClO3-based oxygen generator has been demonstrated experimentally and numerically. Since Al/Mg additive is more energetic than Fe (see Table 1), steady combustion requires a smaller concentration of Al/Mg as compared with Fe. Note also that, in contrast with Mg, mechanically alloyed Al/Mg powder is expected to have much better storage characteristics. Thus, the use of this powder in chemical oxygen generators is worth of further consideration.
6. Conclusions
Thermodynamic calculations for combustion of sodium chlorate mixed with metals and various nanocomposite and mechanically alloyed reactive materials have identified the additives that can be employed at smaller amounts compared to the currently used iron or tin for providing the same combustion temperatures and oxygen yield.
Experiments on combustion of sodium chlorate-based mixtures with nanoscale Co3O4 catalyst and the most promising energetic additives were conducted in argon environment, using laser ignition. Infrared video recording was used to investigate the thermal wave propagation over the mixture pellet. The experiments have shown that mechanically alloyed Al/Mg (1:1 mass ratio) material is a promising alternative to iron and tin. Significantly smaller amounts of this additive, compared to iron, are needed for a steady propagation of the combustion wave and respective steady oxygen generation.
Experiments on combustion of sodium chlorate-based mixtures with nanoscale Co3O4 catalyst and the most promising energetic additives were conducted in argon environment, using laser ignition. Infrared video recording was used to investigate the thermal wave propagation over the mixture pellet. The experiments have shown that mechanically alloyed Al/Mg (1:1 mass ratio) material is a promising alternative to iron and tin. Significantly smaller amounts of this additive, compared to iron, are needed for a steady propagation of the combustion wave and respective steady oxygen generation.
Acknowledgment
This research was supported by the U.S. Department of Defense (Grant No. W911NF-12-1-0056; Grant Officer’s Representative: Dr. Ralph A. Anthenien of the Army Research Office; Co-GOR: Dr. Clifford D. Bedford of the Office of Naval Research).