HYDROGEN GENERATION FROM AMMONIA BORANE AND WATER THROUGH THE COMBUSTION REACTIONS WITH MECHANICALLY ALLOYED AL/MG POWDER
Daniel A. Rodriguez, Edward L. Dreizin, and Evgeny Shafirovich
Finding and developing a safe and effective method for hydrogen storage is integral to its use as an alternative source of energy. The goal of the studies described was to investigate the feasibility of developing combustible hydrogen-generating compositions based on ammonia borane and novel energetic materials such as nanocomposite and mechanically alloyed reactive materials, recently obtained by Prof. Edward Dreizin’s team at the New Jersey Institute of Technology (NJIT). Such compositions could be stored for long time and release hydrogen on demand, upon ignition. The first phase of the research included thermodynamic calculations for combustion of ammonia borane with various reactive materials obtained at NJIT. The second phase involved experiments with compositions that appeared to be promising based on thermodynamic calculations. An experimental setup with laser ignition of mixtures was developed for these experiments. As a result of these tests, further work was focused on mixtures of ammonia borane, gelled water, and mechanically alloyed Al/Mg powder. The last part of the research revealed the reaction mechanisms during combustion of these mixtures. For this purpose, isotopic tests, involving use of heavy water and mass-spectroscopy of gaseous combustion products, were conducted. The results of the present work indicate that combustible mixtures of ammonia borane, water, and mechanically alloyed Al/Mg powder are promising for the development of hydrogen generators that release large amounts of hydrogen upon ignition.
1. Introduction
The overarching goal of the proposed research is to develop application-customized chemical gas generators based on novel energetic materials that will exhibit improved effectiveness, process stability, and fire safety. Chemical gas generators typically include a solid compound that decomposes at increased temperatures and various heat-generating additives. The development of efficient, compact and reliable energy storage system based on hydrogen production represents a challenge. Several hydrogen generating compositions such as ammonia borane or sodium borohydride have been studied. The compositions also include a reactive material or an oxidizer that would produce hydrogen from the hydrogen source by thermolysis, hydrolysis or both. As a result, hydrogen is generated through a self-sustained propagation of the reaction wave over the generator’s chemical core. The project objective is to determine the characteristics and reaction mechanisms of gas-generating compositions involving novel nanocomposite and mechanically alloyed reactive materials, produced by arrested reactive milling, a technique developed recently at the New Jersey Institute of Technology (NJIT).
2. Problem and Purpose Statement
The requirement for efficient and safe methods for hydrogen storage is a major problem that must be overcome to allow the use of hydrogen as an alternative energy carrier. Even though many hydride complexes have certain features that might be attractive for chemical hydrogen storage, the large hydrogen capacities needed for transportation applications exclude most compounds. A promising compound such as ammonia borane (AB, NH3BH3) contains 19.6 wt% H2, as well as possesses storage stability and non-toxicity. Therefore, AB has a remarkable potential for storing and delivering large amounts of hydrogen through dehydrogenation reactions. Despite the fact that NH3BH3 has a negative formation enthalpy (-178 kJ/mol), it is not able to maintain a self-sustained combustion due to heat losses; therefore, energetic additives need to be added. Previous work has shown that hydrogen can be released from AB hydrolytically and thermolytically. These processes are further discussed in Chapter 2 and 3. The requirements for 2 hydrogen release for utilization in fuel cells are fast, controlled, and complete release. Thus, in order to achieve a high energy material weight percent, the amount of AB has to be maximized and most of the hydrogen needs to be released or the hydrogen yield will be low. The energetic additives proposed include metal-metal, metal-metalloid, and metal-oxide (thermite) reactive nanocomposites as well as mechanically alloyed materials. These new energetic materials could produce the required heat for achieving a complete thermolysis process of AB. It was hypothesized that with these materials, hydrogen generators could display improved effectiveness. Testing this hypothesis requires measurements of the amount of energetic additive needed for having a self-sustained combustion process, the combustion front velocity, as well as the hydrogen yield. Comparison of the combustion characteristics for different energetic additives permits one to analyze the potential effect that they could provide for the production of hydrogen from AB.
3. Overview of Methodology
Initial selection of the most promising energetic additive candidates was based on thermodynamic calculations of the adiabatic flame temperatures and product compositions. Then the mixtures were compared based on the amount of additive needed for achieving the desired temperatures for decomposing AB. Once the best candidates were selected, experiments were conducted. The first step of the experimental procedure was to test if the suggested energetic additives were reliable. Therefore, experiments were conducted for the most promising additives. As a result of these experiments, a mixture of AB, gelled water and mechanically alloyed Al/Mg powder was selected for a detailed investigation. The second step was to analyze the combustion characteristics and measuring the hydrogen yield via different methods described further below. Combustion experiments were conducted by making a pellet or a water-gelled mixture composed of the hydrogen source (NH3BH3) and the desired energetic additive. Diagnostics were provided by a high-resolution video camera for visualization of the combustion wave propagation and measurement of the combustion front velocity. The pressure increase due to the gas formation was measured using a pressure transducer, which allowed calculating (using the ideal gas equation) the mass of produced hydrogen. The hydrogen yield was also calculated using mass spectroscopy and then compared with the results acquired from the pressure increase. Isotopic tests (deuterium) were used for revealing the reaction mechanisms.
4. Thermodynamic Calculations
Thermodynamic calculations of the adiabatic combustion temperatures and combustion product compositions were conducted using the software THERMO (version 4.3), which is based on the Gibbs free energy minimization and contains a database of approximately 3,000 compounds. Of specific importance is the capability of this code to handle both intermetallic and thermite reactions in addition to conventional gas-phase combustion reactions. For each mixture, calculations were conducted over a wide range of AB-to-additive mixture ratios. The mass ratio of the additive components in the calculations corresponded to the maximum adiabatic flame temperature in the additive taken alone (i.e., with no ammonia borane). Pressure was equal to 1 atm in all calculations.
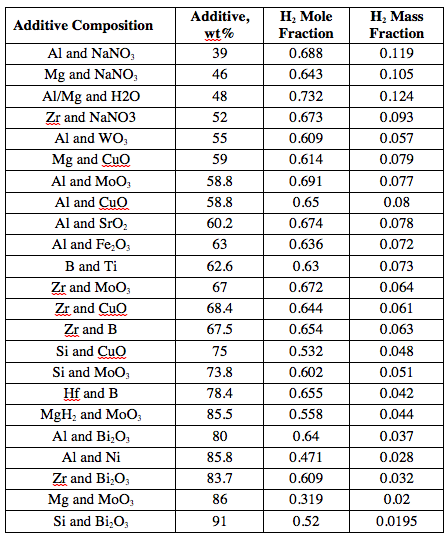
Table 1 shows the amounts of 22 binary mixtures that, being added to ammonia borane, provide the adiabatic flame temperature of 1500°C, which is required for the full decomposition of NH3BH3 to hydrogen and boron nitride. The table also shows the mole and mass fractions of H2 in the combustion products. The order in the table corresponds to increasing the additive mass fraction in the composition. It is seen that the additives that are more efficient in increasing the temperature are usually also more efficient in increasing the hydrogen yield from the unit mass of the entire composition.
Table 1 shows the amounts of 22 binary mixtures that, being added to ammonia borane, provide the adiabatic flame temperature of 1500°C, which is required for the full decomposition of NH3BH3 to hydrogen and boron nitride. The table also shows the mole and mass fractions of H2 in the combustion products. The order in the table corresponds to increasing the additive mass fraction in the composition. It is seen that the additives that are more efficient in increasing the temperature are usually also more efficient in increasing the hydrogen yield from the unit mass of the entire composition.
Table 1. The amounts of additives to ammonia borane that provide the adiabatic flame temperature of 1500°C and the values of H2 mole and mass fractions in the combustion products for these compositions.
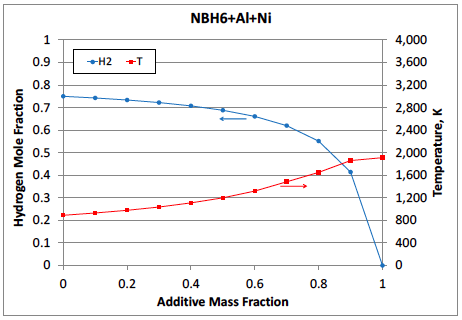
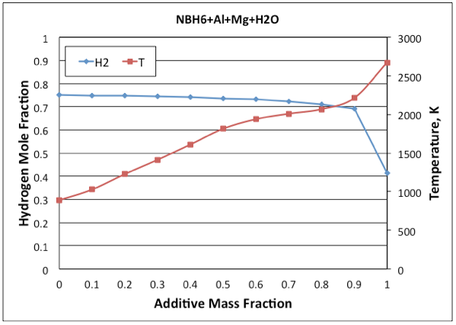
As an example, Figure 1 and 2 shows the calculated adiabatic flame temperatures and hydrogen mole fraction in the combustion products vs the additive mass fraction for the mixtures of ammonia borane with two additives: Al/Ni and Al/Mg/H2O. It is seen that the latter additive is much more effective in increasing the combustion temperature and maintaining a high hydrogen yield. For NBH6/Al/Ni mixture, the desired temperature of 1500°C (1773 K) is reached at 87 wt% Al/Ni additive, which decreases the hydrogen mole fraction in the products from 0.75 (for pure ammonia borane) to 0.45. For NBH6/Al/Mg/H2O mixture, the same temperature is reached at a much smaller additive of Al/Mg/H2O (48 wt%), while the hydrogen mole fraction remains very high (0.73). The calculated adiabatic flame temperatures and equilibrium product compositions can be used for preliminary selection of the additives that are effective in simultaneously providing heat for a selfsustained combustion and maintaining high yield of the desired gas. Since the actual combustion system may be far away from equilibrium, experimental verification is required.
5. Nanocomposite thermites and metal-metalloid composites results
Mixtures based on ammonia borane (BH3NH3) were studied as hydrogen-generating compositions. Based on thermodynamic calculations decsribed in the previous section, the following reactive nanocomposites were tested as energetic additives to ammonia borane: Ti/B, Al/NaNO3, and Al/MoO3. The mixtures were prepared using the tumbler mixer and compacted into pellets. Laser ignition experiments with the mixtures of BH3NH3 and these additives have shown that for successful ignition, the additive concentrations should be as high as 80 wt%, i.e., significantly higher that the optimal values obtained by thermodynamic calculations (30-50 wt% depending on the system). At such high amounts of the additive, hydrogen yield per unit mass of the mixture is low, making the addition of these materials impractical. The unsuccessful experimental results are apparently associated with the large size of ammonia borane particles. Figure 7 and 8 show the particle size distribution for un-milled and milled ammonia borane powder. Ammonia borane was milled in a planetary ball mill (Fritsch Pulverisette 7 Premium Line) at a rotation speed of 750 rpm (the milling time: 5 min; the balls-mixture mass ratio: 10). The average particle size for unmilled AB is 351 μm, while for milled powder it is 183 μm. In both cases, the particle size is very large compared with the energetic additives, for which particle sizes are <50 μm. Since the particle size difference between AB and the additive was large, the area of contact between the mixture components was low, thus making it impossible to ignite.
6. Mechanically alloyed Al/Mg powder with water results
6.1 Preparation of Mixtures
Mechanically alloyed Al·Mg (1:1 mass ratio) powder was prepared in a planetary ball mill (Retsch PM-400 MA) equipped with an air conditioner that cools the milling compartment. Starting materials included elemental powders of Al (Atlantic Equipment Engineers, 99.8% pure, −325 mesh) and Mg (Alfa-Aesar, 99.8% pure, −325 mesh). The Al·Mg powder was prepared following a two-stage procedure. Powders of Al and Mg and 9.5-mm diameter hardened steel balls were loaded in steel milling vials in argon. The powder charge was 30 g per vial and the ball-to-powder mass ratio was 10. Hexane (50 mL) was added to each milling vial as a process control agent (PCA). Milling time was 120 min. The rotation speed was set at 350 rpm and the rotation direction changed every 15 min.
The first stage produced a coarse, mechanically alloyed powder. The second stage of milling, aimed to reduce the particle size, involved the addition of a new PCA, iodine (I2, chips, Sigma Aldrich, 99% pure), at 4 wt% of the initial powder load. The 9.5-mm balls were removed and replaced with the same mass of 3-mm hardened steel balls. The duration of the second milling stage varied between 65 and 125 min, depending on the effectiveness of the air-conditioner cooling which was affected by the air humidity.
One important issue for applications is stability of the prepared materials in aqueous mixtures. Reaction of the mechanically alloyed Al·Mg powder with hot water was investigated with a setup that was previously used in the kinetic studies of the reaction between activated Al powder and water. The setup includes a digital hotplate (Scilogex MS7-H550-Pro) and a system for measuring the volume of released gas based on water displacement in an inverted graduated cylinder. A sample (0.7 g) of the Al·Mg powder was submerged in 750 mL of deionized water at 80 °C. After 24 hours of heating, no reaction was detected. Apparently, the formation of an oxide film on the particles prevented further oxidation. This implies that the mixtures of this powder with water may remain stable for a long time.
The obtained mechanically alloyed Al·Mg powder was mixed with deionized water. To prevent sedimentation of particles, water was gelified by adding polyacrylamide (PAM, linear formula (C3H5NO)n, mass average molecular mass 5·106−6·106, Sigma Aldrich). First, the gellant was added to water and mixed manually for several minutes. Then the Al·Mg powder was mixed (also manually) with the obtained gel. The mass fraction of water in the metal-water mixture was varied from 10 to 60%. A sample of the resulting mixture was then placed in a quartz tube (inner diameter 7.5 mm, thickness 1 mm, height 25 mm) for the combustion experiments.
In the mixtures that contained both mechanically alloyed Al·Mg powder and ammonia borane, heavy water (D2O, Sigma Aldrich, 99.9 at% D), was used. First, D2O was mixed with PAM. Then the obtained gel was mixed with the Al·Mg powder and ammonia borane using an acoustic mixer (Resodyne LabRAM). The mixture compositions are described in Section 3.2. Mixing for 1 min at 50% maximum intensity produced a uniform mixture. A sample (1.1−1.2 g) of the obtained mixture was then placed in a quartz tube (same dimensions as shown above) with the addition of a booster pellet (0.2−0.3 g) on top of the sample. The booster pellet was a stoichiometric mixture of D2O and mechanically alloyed Al·Mg powder. It was used for facilitating ignition of the main mixture.
The first stage produced a coarse, mechanically alloyed powder. The second stage of milling, aimed to reduce the particle size, involved the addition of a new PCA, iodine (I2, chips, Sigma Aldrich, 99% pure), at 4 wt% of the initial powder load. The 9.5-mm balls were removed and replaced with the same mass of 3-mm hardened steel balls. The duration of the second milling stage varied between 65 and 125 min, depending on the effectiveness of the air-conditioner cooling which was affected by the air humidity.
One important issue for applications is stability of the prepared materials in aqueous mixtures. Reaction of the mechanically alloyed Al·Mg powder with hot water was investigated with a setup that was previously used in the kinetic studies of the reaction between activated Al powder and water. The setup includes a digital hotplate (Scilogex MS7-H550-Pro) and a system for measuring the volume of released gas based on water displacement in an inverted graduated cylinder. A sample (0.7 g) of the Al·Mg powder was submerged in 750 mL of deionized water at 80 °C. After 24 hours of heating, no reaction was detected. Apparently, the formation of an oxide film on the particles prevented further oxidation. This implies that the mixtures of this powder with water may remain stable for a long time.
The obtained mechanically alloyed Al·Mg powder was mixed with deionized water. To prevent sedimentation of particles, water was gelified by adding polyacrylamide (PAM, linear formula (C3H5NO)n, mass average molecular mass 5·106−6·106, Sigma Aldrich). First, the gellant was added to water and mixed manually for several minutes. Then the Al·Mg powder was mixed (also manually) with the obtained gel. The mass fraction of water in the metal-water mixture was varied from 10 to 60%. A sample of the resulting mixture was then placed in a quartz tube (inner diameter 7.5 mm, thickness 1 mm, height 25 mm) for the combustion experiments.
In the mixtures that contained both mechanically alloyed Al·Mg powder and ammonia borane, heavy water (D2O, Sigma Aldrich, 99.9 at% D), was used. First, D2O was mixed with PAM. Then the obtained gel was mixed with the Al·Mg powder and ammonia borane using an acoustic mixer (Resodyne LabRAM). The mixture compositions are described in Section 3.2. Mixing for 1 min at 50% maximum intensity produced a uniform mixture. A sample (1.1−1.2 g) of the obtained mixture was then placed in a quartz tube (same dimensions as shown above) with the addition of a booster pellet (0.2−0.3 g) on top of the sample. The booster pellet was a stoichiometric mixture of D2O and mechanically alloyed Al·Mg powder. It was used for facilitating ignition of the main mixture.
6.2 Combustion Experiments
Prepared mixtures were ignited using a CO2 laser. After ignition, the combustion front propagated downward through the
sample. A digital video camera (Sony XCD-SX90CR) recorded the experiment. The pressure increase due to the released
gases was recorded with a pressure transducer (Omegadyne PX-409-030AI).
Mass-spectroscopic analysis of the released gases was performed after cooling to room temperature. To enable quantitative analysis, the mass-spectrometer was calibrated using hydrogen (H2, 99.999% pure, Airgas), deuterium hydride (HD, 96 mol% HD, 98 at% D, Sigma Aldrich), and deuterium (D2, 99.8 at% D, Sigma Aldrich). Condensed products were characterized using X-ray diffraction analysis (Bruker D8 Discover XRD).
Mass-spectroscopic analysis of the released gases was performed after cooling to room temperature. To enable quantitative analysis, the mass-spectrometer was calibrated using hydrogen (H2, 99.999% pure, Airgas), deuterium hydride (HD, 96 mol% HD, 98 at% D, Sigma Aldrich), and deuterium (D2, 99.8 at% D, Sigma Aldrich). Condensed products were characterized using X-ray diffraction analysis (Bruker D8 Discover XRD).
7. Results and Discussion
7.1
Mixtures of mechanically alloyed Al·Mg powder and water
The attempts to ignite stoichiometric mixtures of mechanically alloyed Al·Mg powder and water with no gellant were unsuccessful. Thickening water with 1 wt% polyacrylamide made the mixtures combustible over a range of water concentrations from 20 to 60 wt%. The combustion was self-sustained, i.e., the front propagated after turning off the laser, and the laser beam energy input was about 80 J, while the released heat of combustion was several kJ.
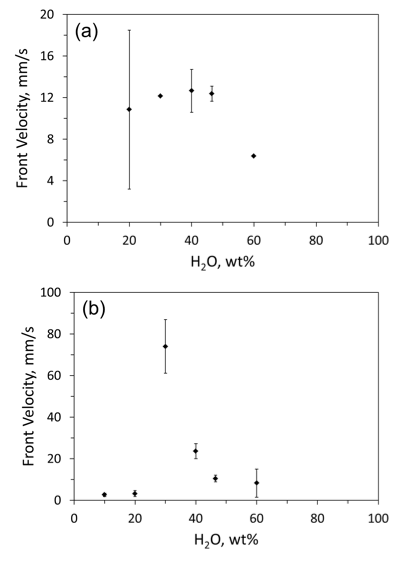
Figure 4 shows average velocities of the combustion front measured in mixtures with 1 and 3 wt% PAM. Here and throughout the paper, the percentage of polyacrylamide is given with respect to water. Each test was repeated three times and the error bars show standard deviation for the observed flame velocities. At 3 wt% PAM, a sharp peak in the flame velocity as a function of water concentration is observed. At less than 20 wt% H2O or more than 40 wt% H2O, the front velocity was relatively low . At 30-40 wt% H2O, the velocity was much higher . The peak velocity observed at 30 wt% of H2O with 3 wt % PAM was consistently observed in three repeated tests. The accelerating effect of polyacrylamide on the combustion may be associated with its decomposition and possible formation of chelate compounds that inhibit growth of a protective oxide film on the metal surface.
The measured combustion front velocities were compared with the data obtained for stoichiometric nano-Al−H2O compositions at a pressure of 1 atm (or 0.1 MPa) where water was gelled with 3 wt% PAM. The comparison shows that the front velocity for the mechanically alloyed Al·Mg powder significantly exceeds those for 120-nm Al powder (2 mm/s) and 38-nm Al powder (7 mm/s). It is important that the 38-nm powder had an active aluminum content of only about 54.3 wt%, while the fraction of oxidized metal in the mechanically alloyed Al·Mg powder is not expected to exceed 2 wt%.
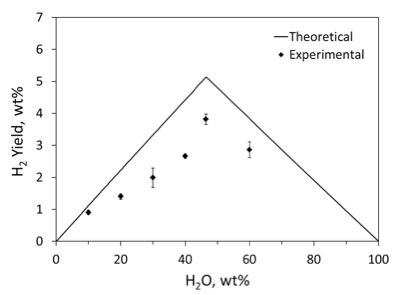
Figure 5 shows the values of hydrogen yield, calculated from the pressure increase measured after combustion and cooling, with the assumption that the released gas is pure hydrogen. For comparison, the figure also shows theoretical values calculated with assumption of complete conversion of the following reactions:
2Al+3H2O →Al2O3+3H2 (1)
Mg+H2O →MgO+ H2 (2)
It is seen that most experimental values exceed the theoretical ones, and the difference increases with increased concentration of polyacrylamide. These observations are explained by PAM decomposition, which generates gases and increases pressure in the chamber. Note that flash pyrolysis of PAM at 700 °C led to the formation of 24 organic products. Such a variety of possible products makes it difficult to estimate the pressure increase based on the mass of decomposed PAM for comparison with the experimental data. Mass-spectroscopic analysis of the gas environment in the chamber after combustion has detected additional peaks along with those of argon, hydrogen, and air traces, which confirms the presence of gases evolved from polyacrylamide.
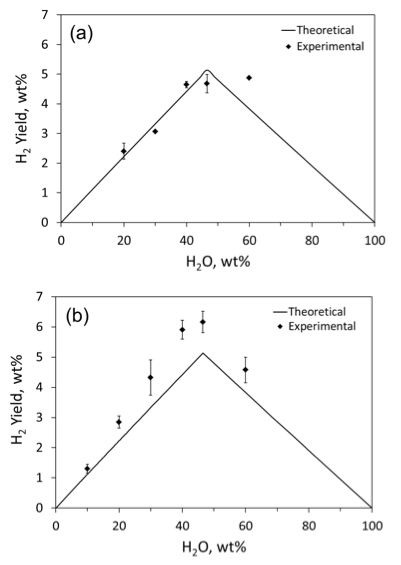
Further mass-spectroscopic analysis was focused on the measurements of hydrogen concentration. Using binary Ar/H2 gas mixtures with different hydrogen contents, the mass-spectrometer was calibrated to measure H2 concentration in the gas environment after combustion. Figure 6 shows the obtained values of hydrogen yield in comparison with theoretical values. It is seen that the efficiency of hydrogen release from the stoichiometric mixture is about 80%. Apparently, part of water escaped from the mixture because of vaporization during laser heating and as an unreacted water vapor during the combustion front propagation
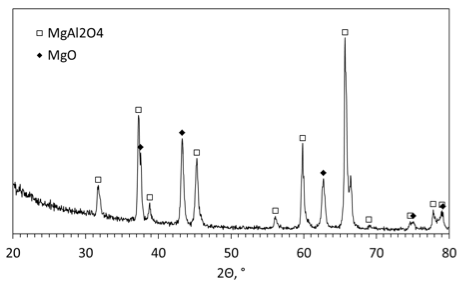
Figure 7 presents an XRD pattern of the solid products collected after combustion of mechanically alloyed Al·Mg powder with water at 46.5 wt% water. This concentration corresponds to the stoichiometric ratios for reactions (Eqs. 1 and 2). The identified compounds in the products are MgAl2O4 and MgO. The absence of Mg peaks indicates that the reaction of Mg with water was complete (within the limits of XRD accuracy). According to the XRD database, the peaks of Al overlap with those of MgAl2O4 so that no conclusion about the presence of Al in the products can be made from the XRD pattern.
Figure 4 shows average velocities of the combustion front measured in mixtures with 1 and 3 wt% PAM. Here and throughout the paper, the percentage of polyacrylamide is given with respect to water. Each test was repeated three times and the error bars show standard deviation for the observed flame velocities. At 3 wt% PAM, a sharp peak in the flame velocity as a function of water concentration is observed. At less than 20 wt% H2O or more than 40 wt% H2O, the front velocity was relatively low . At 30-40 wt% H2O, the velocity was much higher . The peak velocity observed at 30 wt% of H2O with 3 wt % PAM was consistently observed in three repeated tests. The accelerating effect of polyacrylamide on the combustion may be associated with its decomposition and possible formation of chelate compounds that inhibit growth of a protective oxide film on the metal surface.
The measured combustion front velocities were compared with the data obtained for stoichiometric nano-Al−H2O compositions at a pressure of 1 atm (or 0.1 MPa) where water was gelled with 3 wt% PAM. The comparison shows that the front velocity for the mechanically alloyed Al·Mg powder significantly exceeds those for 120-nm Al powder (2 mm/s) and 38-nm Al powder (7 mm/s). It is important that the 38-nm powder had an active aluminum content of only about 54.3 wt%, while the fraction of oxidized metal in the mechanically alloyed Al·Mg powder is not expected to exceed 2 wt%.
Figure 5 shows the values of hydrogen yield, calculated from the pressure increase measured after combustion and cooling, with the assumption that the released gas is pure hydrogen. For comparison, the figure also shows theoretical values calculated with assumption of complete conversion of the following reactions:
2Al+3H2O →Al2O3+3H2 (1)
Mg+H2O →MgO+ H2 (2)
It is seen that most experimental values exceed the theoretical ones, and the difference increases with increased concentration of polyacrylamide. These observations are explained by PAM decomposition, which generates gases and increases pressure in the chamber. Note that flash pyrolysis of PAM at 700 °C led to the formation of 24 organic products. Such a variety of possible products makes it difficult to estimate the pressure increase based on the mass of decomposed PAM for comparison with the experimental data. Mass-spectroscopic analysis of the gas environment in the chamber after combustion has detected additional peaks along with those of argon, hydrogen, and air traces, which confirms the presence of gases evolved from polyacrylamide.
Further mass-spectroscopic analysis was focused on the measurements of hydrogen concentration. Using binary Ar/H2 gas mixtures with different hydrogen contents, the mass-spectrometer was calibrated to measure H2 concentration in the gas environment after combustion. Figure 6 shows the obtained values of hydrogen yield in comparison with theoretical values. It is seen that the efficiency of hydrogen release from the stoichiometric mixture is about 80%. Apparently, part of water escaped from the mixture because of vaporization during laser heating and as an unreacted water vapor during the combustion front propagation
Figure 7 presents an XRD pattern of the solid products collected after combustion of mechanically alloyed Al·Mg powder with water at 46.5 wt% water. This concentration corresponds to the stoichiometric ratios for reactions (Eqs. 1 and 2). The identified compounds in the products are MgAl2O4 and MgO. The absence of Mg peaks indicates that the reaction of Mg with water was complete (within the limits of XRD accuracy). According to the XRD database, the peaks of Al overlap with those of MgAl2O4 so that no conclusion about the presence of Al in the products can be made from the XRD pattern.
Fig. 3. Combustion of Al·Mg−H2O mixture at 20 wt% H2O. Time zero was selected arbitrarily.
|
Fig. 4. Combustion front velocities in Al·Mg−water mixtures vs water concentration, at (a) 1 wt% and (b) 3 wt% polyacrylamide.
Fig. 6. Hydrogen yield determined by mass-spectrometry for Al·Mg−water mixtures vs water concentration at 3 wt% polyacrylamide, in comparison with theoretical values.
|
Fig. 5. Hydrogen yield determined from pressure measurements for Al·Mg−water mixtures vs water concentration at (a) 1 wt% and (b) 3 wt% polyacrylamide, in comparison with theoretical values.
Fig. 7. XRD pattern of combustion products for the stoichiometric mixture of mechanically alloyed Al·Mg powder and water.
|
7.2
Mixtures of ammonia borane, mechanically alloyed Al·Mg
powder, and water
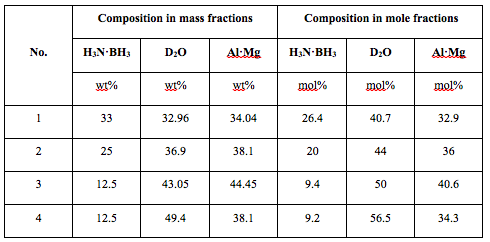
To enable isotopic tests, combustion experiments with the mixtures were conducted using D2O instead of H2O. Table 2 shows compositions of the tested mixtures, both in mass and mole fractions. Note that use of D2O instead of H2O changes the mass fractions, but does not change the mole ratio. Mixtures 1, 2, and 3 were designed based on the assumption that AB decomposes thermally, while the amount of water (in moles) corresponds to the stoichiometric reactions with Al and Mg (Eqs. 1 and 2). Mixture 4 included more water to enable hydrolysis of AB while maintaining enough water for the reaction with Al·Mg.
Table 2. Tested mixture compositions
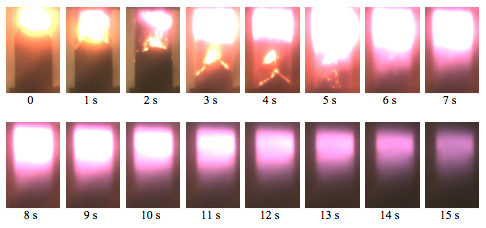
The attempts to ignite mixture 1 were unsuccessful, while three other mixtures were combustible. Figure 8 shows combustion of mixture 3. The first and second images correspond to combustion of the booster pellet. The next four images show propagation of the combustion front over the main mixture. They also show formation of a wide glowing zone at the sample top, i.e., behind the front. This zone continued to emit light for several seconds after the front reached the bottom. Although this zone was located at the top, it could not be related to the booster pellet because its solid combustion products (oxides of Al and Mg) were ejected by the released gas from the tube. The formation and continuous glowing of a wide luminous zone was observed in all experiments with mixtures that contained AB. On the other hand, this phenomenon was not observed in the experiments with Al·Mg−H2O. Apparently, the glowing is associated with ammonia borane or its products. The rates of processes that involve AB are probably lower than the burn rate of Al·Mg particles with H2O. Note that multi-zone reaction waves have been observed in many combustion systems, particularly, in self-propagating high-temperature synthesis (SHS).
Fig. 8. Combustion of mixture 3. Time zero was selected arbitrarily.
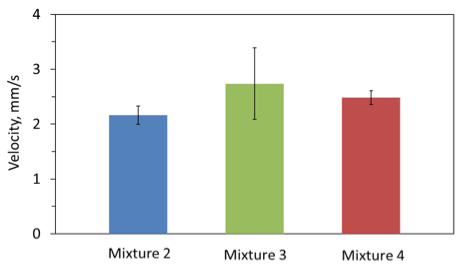
Figure 9 shows the combustion front velocities for mixtures 2, 3, and 4. Each point was obtained based on the results of three tests. It is seen that mixture 2, with the highest concentration of AB, has the lowest velocity. Also, comparison with the data for Al·Mg−water mixtures (Fig. 2) shows that the addition of AB significantly decreases the front velocity. This is not unexpected because the heat release values of AB thermolysis and hydrolysis are much smaller than the heat release of Al·Mg reaction with water. Further, mixture 4 exhibits a lower front velocity than mixture 3. This is apparently explained by a lower combustion temperature in mixture 4 due to the larger amount of water there.
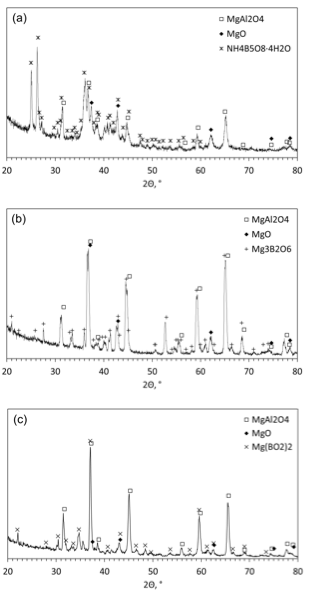
Figure 10 shows XRD patterns of solid products obtained after combustion of mixtures 2, 3, and 4. All three patterns indicate the presence of MgAl2O4 and MgO, produced by the reaction between Al·Mg particles and water. Also, in each pattern, a compound that contains a borate anion is detected: ammonium pentaborate tetrahydrate NH4B5O8·4H2O for mixture 2, magnesium orthoborate Mg3(BO3)2 for mixture 3, and magnesium metaborate Mg(BO2)2 for mixture 4. Note that, since heavy water was used in these experiments, the actual formula of detected ammonium pentaborate tetrahydrate probably contains D2O instead of H2O. The presence of a borate anion in each pattern is explained by AB hydrolysis. A type of cation bound with this anion depends on the mixture composition: it is ammonium for mixture 2 and magnesium for mixtures 3 and 4. The presence of ammonium cation in the products of mixture 2 is apparently associated with the higher concentration of AB in this mixture. The absence of BN in any pattern indicates that the third step of AB decomposition was not achieved. The condensed products of the first and second stages of AB thermolysis are amorphous according to the literature and hence they cannot be seen in XRD patterns.
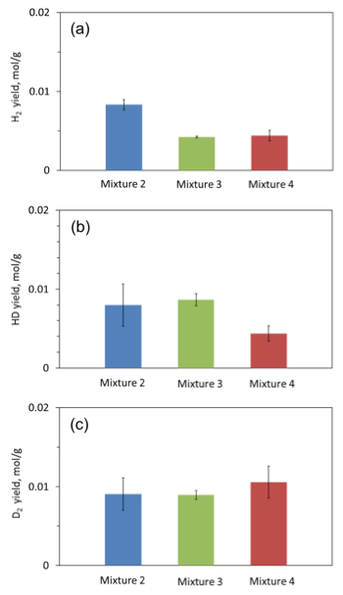
The concentrations of H2, HD, and D2 in the gaseous products of each mixture were determined by mass-spectroscopic analysis. Figure 11 show the measured amounts of H2, HD, and D2 per unit mass of the sample. Note that each sample included a booster pellet, which slightly decreased the difference between the results obtained for different mixtures. Each value in Fig. 11 was obtained based on three tests. It is seen that each mixture generates comparable concentrations of the three gases. This clearly indicates that all three possible pathways for hydrogen release take place: thermolysis of H3NBH3 (produces H2), reaction between H3NBH3 and D2O (produces HD), and reaction between Al·Mg and D2O (produces D2). Mixture 2 produced twice more H2 than mixtures 3 and 4. This corresponds to the twofold content of AB in mixture 2. Despite the lower content of AB, however, mixture 3 produced as much HD as mixture 2. This indicates that hydrolysis increased the efficiency of using AB in this mixture. The largest amount of D2 was obtained from mixture 4. This is understandable because in mixtures 2 and 3 there was not enough D2O for Al·Mg combustion because part of D2O was consumed by AB hydrolysis, while mixture 4 had a twofold amount of D2O. On the other hand, the amount of HD for mixture 4 was less than for mixture 3. This is apparently explained by the lower hydrolysis reaction rate due to the lower combustion temperature in mixture 4.
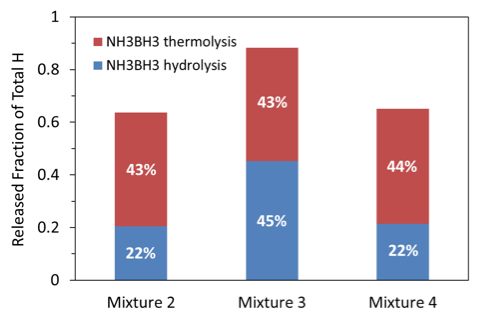
For better clarity, based on the measured amounts of H2 and HD, a plot was generated (Fig. 12) that shows, for each mixture, the fraction of the total available H that was detected in gas phase. The total available amount of H in the calculations was determined for each sample based on the actual mass of H3N·BH3 in the sample. In addition, the same figure shows what percentage of the produced H came from thermal decomposition of AB and how much came from AB hydrolysis as “H” part of the formed HD. It is seen that for the mixtures 2 and 4 only 65-66 % of H, available in AB, was released to gas phase and detected as either H2 or part of HD, while for mixture 3, the released fraction of H increased to 88%. The rest of hydrogen apparently remained in the condensed products as part of polymeric boron-hydrogen compounds and ammonium cation (see Fig. 11 and discussion of XRD results). It is also clearly seen in Fig. 10 that the increase in H yield for mixture 3 was caused by the increased role of AB hydrolysis.
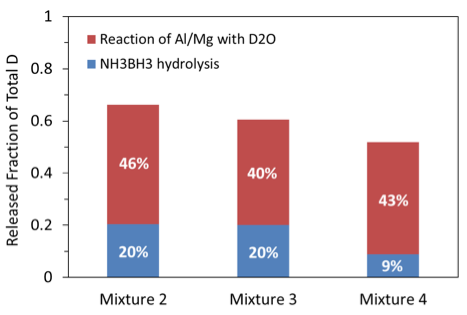
Similarly, based on the measured amounts of HD and D2, a plot was generated (Fig. 13) that shows, for each mixture, the fraction of the total available deuterium that was detected in gas phase as either D2 or part of HD. The total available amount of D in the calculations was determined for each sample based on the actual mass of D2O in the sample. In addition, the same figure shows what percentage of the released deuterium came from the reaction of Al·Mg with water and how much came from AB hydrolysis. It is seen that the maximum fraction of deuterium released to gas phase was only 66% (mixture 2). This is apparently associated with the loss of D2O during ignition and combustion. Indeed, part of vaporized D2O could escape from the sample and then condense in the chamber. The efficiency of releasing D in mixtures 2 and 3 is about the same despite the larger amount of D2O in mixture 3. It is interesting that in mixture 4, the attempt to compensate the loss of heavy water and increase the amount of D2O available for AB hydrolysis resulted in a lower efficiency of D release because of the lower yield from AB hydrolysis, apparently, explained by the lower combustion temperature.
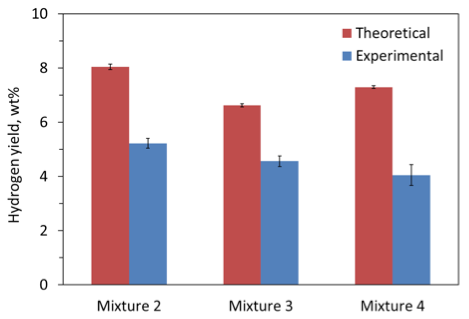
To determine the maximum hydrogen yield that could be achieved for the tested compositions if H2O were used instead of D2O, the measured masses of released H2, HD, and D2 as well as the sample masses were recalculated for H as the only isotope of hydrogen. Figure 14 shows the resulting total hydrogen yield per unit mass of sample for each composition. Note that the recalculation procedure accounted for the fact that each sample included a booster pellet in addition to the main mixture. The maximum theoretical yield for each sample (based on the assumption that all hydrogen is released) is also shown in this figure. Here each sample also included both the main mixture and booster pellet. Standard deviations of the theoretical values were caused by the actual variation of the booster pellet mass fraction in experiments. It is seen that mixture 2 provides the highest hydrogen yield (both experimentally and theoretically), while mixture 3 is the most efficient from the standpoint of approaching the theoretical limit in the experiments.
Comparison of hydrogen yield for mixtures 2 and 3 with that for the mixtures of mechanically alloyed Al·Mg powder with water shows that the addition of AB increases hydrogen production. Tuning the composition and scaling up to a practical hydrogen-generating reactor may further increase hydrogen yield. Concentration of AB could be further increased to provide more hydrogen from AB while keeping a sufficiently high exothermicity of the mixture. A larger diameter of the reactor would decrease the heat losses, leading to a higher combustion temperature and hence to a possibility of adding more AB. Also, an increase in the reactor height may decrease the loss of water during ignition and combustion of the mixture. Finally, for a larger reactor, the mass fraction of the booster pellet would be smaller, leading to a higher hydrogen yield.
Note that compositions 2 and 3 were designed assuming that no hydrolysis of AB occurs and all water is consumed by the reaction with Al·Mg. In reality, AB hydrolysis occurs and consumes some water, shifting the water-metal ratio from the stoichiometry to having more water in the mixture. For the same amount of AB, the production of hydrogen may, therefore, increase. On the other hand, the shift from stoichiometry reduces the combustion temperature, leading to a lower rate of AB hydrolysis and a lower hydrogen yield (see the result for mixture 4). Thus, there should be an optimal concentration where there is enough water for the reactions with both Al·Mg and AB, while the combustion temperature remains sufficiently high. Because this optimization is affected by the reaction temperature, it is also affected by specific heat transfer conditions and thus by the sample mass and shape.
Figure 10 shows XRD patterns of solid products obtained after combustion of mixtures 2, 3, and 4. All three patterns indicate the presence of MgAl2O4 and MgO, produced by the reaction between Al·Mg particles and water. Also, in each pattern, a compound that contains a borate anion is detected: ammonium pentaborate tetrahydrate NH4B5O8·4H2O for mixture 2, magnesium orthoborate Mg3(BO3)2 for mixture 3, and magnesium metaborate Mg(BO2)2 for mixture 4. Note that, since heavy water was used in these experiments, the actual formula of detected ammonium pentaborate tetrahydrate probably contains D2O instead of H2O. The presence of a borate anion in each pattern is explained by AB hydrolysis. A type of cation bound with this anion depends on the mixture composition: it is ammonium for mixture 2 and magnesium for mixtures 3 and 4. The presence of ammonium cation in the products of mixture 2 is apparently associated with the higher concentration of AB in this mixture. The absence of BN in any pattern indicates that the third step of AB decomposition was not achieved. The condensed products of the first and second stages of AB thermolysis are amorphous according to the literature and hence they cannot be seen in XRD patterns.
The concentrations of H2, HD, and D2 in the gaseous products of each mixture were determined by mass-spectroscopic analysis. Figure 11 show the measured amounts of H2, HD, and D2 per unit mass of the sample. Note that each sample included a booster pellet, which slightly decreased the difference between the results obtained for different mixtures. Each value in Fig. 11 was obtained based on three tests. It is seen that each mixture generates comparable concentrations of the three gases. This clearly indicates that all three possible pathways for hydrogen release take place: thermolysis of H3NBH3 (produces H2), reaction between H3NBH3 and D2O (produces HD), and reaction between Al·Mg and D2O (produces D2). Mixture 2 produced twice more H2 than mixtures 3 and 4. This corresponds to the twofold content of AB in mixture 2. Despite the lower content of AB, however, mixture 3 produced as much HD as mixture 2. This indicates that hydrolysis increased the efficiency of using AB in this mixture. The largest amount of D2 was obtained from mixture 4. This is understandable because in mixtures 2 and 3 there was not enough D2O for Al·Mg combustion because part of D2O was consumed by AB hydrolysis, while mixture 4 had a twofold amount of D2O. On the other hand, the amount of HD for mixture 4 was less than for mixture 3. This is apparently explained by the lower hydrolysis reaction rate due to the lower combustion temperature in mixture 4.
For better clarity, based on the measured amounts of H2 and HD, a plot was generated (Fig. 12) that shows, for each mixture, the fraction of the total available H that was detected in gas phase. The total available amount of H in the calculations was determined for each sample based on the actual mass of H3N·BH3 in the sample. In addition, the same figure shows what percentage of the produced H came from thermal decomposition of AB and how much came from AB hydrolysis as “H” part of the formed HD. It is seen that for the mixtures 2 and 4 only 65-66 % of H, available in AB, was released to gas phase and detected as either H2 or part of HD, while for mixture 3, the released fraction of H increased to 88%. The rest of hydrogen apparently remained in the condensed products as part of polymeric boron-hydrogen compounds and ammonium cation (see Fig. 11 and discussion of XRD results). It is also clearly seen in Fig. 10 that the increase in H yield for mixture 3 was caused by the increased role of AB hydrolysis.
Similarly, based on the measured amounts of HD and D2, a plot was generated (Fig. 13) that shows, for each mixture, the fraction of the total available deuterium that was detected in gas phase as either D2 or part of HD. The total available amount of D in the calculations was determined for each sample based on the actual mass of D2O in the sample. In addition, the same figure shows what percentage of the released deuterium came from the reaction of Al·Mg with water and how much came from AB hydrolysis. It is seen that the maximum fraction of deuterium released to gas phase was only 66% (mixture 2). This is apparently associated with the loss of D2O during ignition and combustion. Indeed, part of vaporized D2O could escape from the sample and then condense in the chamber. The efficiency of releasing D in mixtures 2 and 3 is about the same despite the larger amount of D2O in mixture 3. It is interesting that in mixture 4, the attempt to compensate the loss of heavy water and increase the amount of D2O available for AB hydrolysis resulted in a lower efficiency of D release because of the lower yield from AB hydrolysis, apparently, explained by the lower combustion temperature.
To determine the maximum hydrogen yield that could be achieved for the tested compositions if H2O were used instead of D2O, the measured masses of released H2, HD, and D2 as well as the sample masses were recalculated for H as the only isotope of hydrogen. Figure 14 shows the resulting total hydrogen yield per unit mass of sample for each composition. Note that the recalculation procedure accounted for the fact that each sample included a booster pellet in addition to the main mixture. The maximum theoretical yield for each sample (based on the assumption that all hydrogen is released) is also shown in this figure. Here each sample also included both the main mixture and booster pellet. Standard deviations of the theoretical values were caused by the actual variation of the booster pellet mass fraction in experiments. It is seen that mixture 2 provides the highest hydrogen yield (both experimentally and theoretically), while mixture 3 is the most efficient from the standpoint of approaching the theoretical limit in the experiments.
Comparison of hydrogen yield for mixtures 2 and 3 with that for the mixtures of mechanically alloyed Al·Mg powder with water shows that the addition of AB increases hydrogen production. Tuning the composition and scaling up to a practical hydrogen-generating reactor may further increase hydrogen yield. Concentration of AB could be further increased to provide more hydrogen from AB while keeping a sufficiently high exothermicity of the mixture. A larger diameter of the reactor would decrease the heat losses, leading to a higher combustion temperature and hence to a possibility of adding more AB. Also, an increase in the reactor height may decrease the loss of water during ignition and combustion of the mixture. Finally, for a larger reactor, the mass fraction of the booster pellet would be smaller, leading to a higher hydrogen yield.
Note that compositions 2 and 3 were designed assuming that no hydrolysis of AB occurs and all water is consumed by the reaction with Al·Mg. In reality, AB hydrolysis occurs and consumes some water, shifting the water-metal ratio from the stoichiometry to having more water in the mixture. For the same amount of AB, the production of hydrogen may, therefore, increase. On the other hand, the shift from stoichiometry reduces the combustion temperature, leading to a lower rate of AB hydrolysis and a lower hydrogen yield (see the result for mixture 4). Thus, there should be an optimal concentration where there is enough water for the reactions with both Al·Mg and AB, while the combustion temperature remains sufficiently high. Because this optimization is affected by the reaction temperature, it is also affected by specific heat transfer conditions and thus by the sample mass and shape.
Fig. 9. Combustion front velocity for each mixture.
|
Fig. 10. XRD pattern of the products obtained after combustion of mixtures (a) 2, (b) 3, and (c) 4.
Fig. 12. Released fraction of total available H via AB thermolysis and AB hydrolysis.
|
Fig. 11. Measured amounts of (a) H2, (b) HD, and (c) D2 per unit mass of the sample.
Fig. 13. Released fraction of total available D via reaction of Al·Mg with D2O and AB hydrolysis.
|
Fig. 14. Theoretical gravimetric hydrogen yield of AB−H2O−Al·Mg mixtures and its experimental values obtained by recalculating the data obtained in experiments with AB−D2O− Al·Mg mixtures.
8. Conclusions
A novel approach to hydrogen release from ammonia borane has been tested that involves the reaction of mechanically alloyed Al·Mg powder with water as a source of heat for AB thermolysis and hydrolysis. This reaction also releases hydrogen from water, thus increasing the total hydrogen yield.
Experiments have shown that mixtures of mechanically alloyed Al·Mg powder with gelled water are combustible. The velocities of combustion front propagation exceed the values obtained for mixtures of nanoscale Al powder with gelled water. At the same time, no reaction occurs between mechanically alloyed Al·Mg powder and hot (80 °C) water for 24 hours, which indicates that the mixtures can remain stable for long time.
Experiments have been conducted with mixtures of AB, mechanically alloyed Al·Mg powder, and heavy water (D2O), where the latter was used for investigating the reaction mechanisms through mass-spectroscopy of released H2, HD, and D2 gases (isotopic tests). The addition of ammonia borane to the Al·Mg−water mixture increased the total hydrogen yield. The isotopic tests have shown that AB participates in two parallel processes − thermolysis and hydrolysis. Because of this, as much as 88% of hydrogen contained in AB was released in one of the tested mixtures, which significantly exceeds the amount released in the first and second steps of AB thermolysis (35-70%). Tuning the composition and scaling up to a practical hydrogen-generating reactor may further increase hydrogen yield in these mixtures.
Experiments have shown that mixtures of mechanically alloyed Al·Mg powder with gelled water are combustible. The velocities of combustion front propagation exceed the values obtained for mixtures of nanoscale Al powder with gelled water. At the same time, no reaction occurs between mechanically alloyed Al·Mg powder and hot (80 °C) water for 24 hours, which indicates that the mixtures can remain stable for long time.
Experiments have been conducted with mixtures of AB, mechanically alloyed Al·Mg powder, and heavy water (D2O), where the latter was used for investigating the reaction mechanisms through mass-spectroscopy of released H2, HD, and D2 gases (isotopic tests). The addition of ammonia borane to the Al·Mg−water mixture increased the total hydrogen yield. The isotopic tests have shown that AB participates in two parallel processes − thermolysis and hydrolysis. Because of this, as much as 88% of hydrogen contained in AB was released in one of the tested mixtures, which significantly exceeds the amount released in the first and second steps of AB thermolysis (35-70%). Tuning the composition and scaling up to a practical hydrogen-generating reactor may further increase hydrogen yield in these mixtures.
Acknowledgment
This research was supported by the U.S. Department of Defense (Grants No. W911NF-12-1-0056 and No. W911NF-14-1-0034; Grant Officer’s Representative: Dr. Ralph A. Anthenien of the Army Research Office; Co-GOR: Dr. Clifford D. Bedford of the Office of Naval Research).